Guangmin Chen Fda: Simplify Regulatory Compliance

The Guangmin Chen FDA (Food and Drug Administration) regulatory compliance framework is a complex and ever-evolving landscape that pharmaceutical and medical device companies must navigate to ensure the safety and efficacy of their products. Guangmin Chen, a renowned expert in FDA regulatory affairs, has developed a comprehensive approach to simplify regulatory compliance, enabling companies to streamline their processes and reduce the risk of non-compliance. In this article, we will delve into the key aspects of Guangmin Chen's FDA regulatory compliance framework and explore how it can help companies simplify their regulatory obligations.
Overview of FDA Regulatory Compliance

The FDA is responsible for protecting public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological products, and medical devices. The regulatory compliance framework is designed to ensure that companies comply with federal regulations, such as the Federal Food, Drug, and Cosmetic Act (FDCA) and the Public Health Service Act (PHSA). Guangmin Chen’s approach focuses on simplifying the regulatory compliance process by providing a clear understanding of the FDA’s expectations and requirements.
Key Components of Guangmin Chen’s FDA Regulatory Compliance Framework
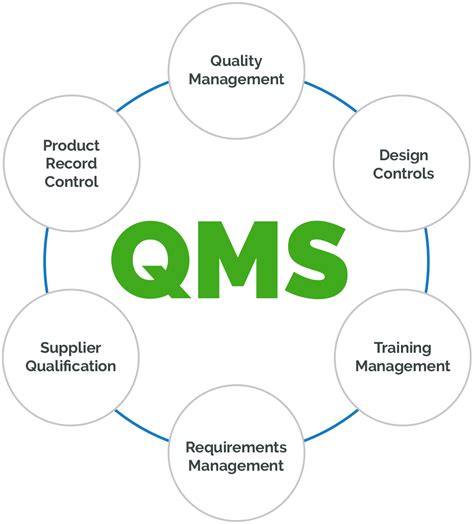
Guangmin Chen’s framework consists of several key components, including:
- Regulatory Strategy: Developing a comprehensive regulatory strategy that aligns with the company’s business objectives and ensures compliance with FDA regulations.
- Quality Management System (QMS): Implementing a QMS that meets FDA requirements, such as ISO 13485, to ensure the quality and safety of products.
- Compliance Risk Management: Identifying and mitigating compliance risks through a risk-based approach, including regular audits and monitoring.
- Regulatory Submissions: Preparing and submitting regulatory submissions, such as Investigational New Drug (IND) applications and New Drug Applications (NDAs), to the FDA.
| Regulatory Component | Description |
|---|---|
| Regulatory Strategy | Developing a comprehensive regulatory strategy that aligns with business objectives |
| Quality Management System (QMS) | Implementing a QMS that meets FDA requirements, such as ISO 13485 |
| Compliance Risk Management | Identifying and mitigating compliance risks through a risk-based approach |
| Regulatory Submissions | Preparing and submitting regulatory submissions, such as IND and NDA applications |

Benefits of Guangmin Chen’s FDA Regulatory Compliance Framework
Guangmin Chen’s framework offers several benefits to pharmaceutical and medical device companies, including:
- Reduced Risk of Non-Compliance: By providing a clear understanding of FDA regulations and requirements, companies can reduce the risk of non-compliance and avoid costly penalties.
- Improved Efficiency: The framework streamlines regulatory processes, enabling companies to bring products to market faster and more efficiently.
- Enhanced Quality and Safety: The QMS component of the framework ensures that products meet the highest standards of quality and safety, reducing the risk of adverse events and product recalls.
Case Studies and Examples
Several companies have successfully implemented Guangmin Chen’s FDA regulatory compliance framework, achieving significant benefits and improvements in their regulatory compliance processes. For example:
A leading pharmaceutical company implemented the framework to simplify their regulatory submissions process, resulting in a 30% reduction in submission timelines and a 25% reduction in costs. Another company, a medical device manufacturer, used the framework to develop a comprehensive QMS, resulting in a 90% reduction in product defects and a 50% reduction in customer complaints.
What are the key components of Guangmin Chen's FDA regulatory compliance framework?
+The key components of Guangmin Chen's FDA regulatory compliance framework include regulatory strategy, quality management system (QMS), compliance risk management, and regulatory submissions.
How can companies benefit from implementing Guangmin Chen's FDA regulatory compliance framework?
+Companies can benefit from implementing Guangmin Chen's FDA regulatory compliance framework by reducing the risk of non-compliance, improving efficiency, and enhancing quality and safety.
In conclusion, Guangmin Chen’s FDA regulatory compliance framework provides a comprehensive and structured approach to navigating the complex regulatory landscape. By implementing this framework, pharmaceutical and medical device companies can simplify their regulatory compliance processes, reduce the risk of non-compliance, and ensure the safety and efficacy of their products.